Zyprexa, Abilify, Risperdal, and Seroquel Linked to Rapid Weight Gain in Children
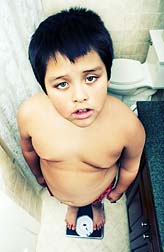
Washington, DCThe largest study of its kind to show an association between atypical antipsychotics such as Zyprexa, and rapid weight gain and metabolic changes that can lead to diabetes and hypertension was published Wednesday in the Journal of the American Medical Association (JAMA). The study was conducted in 272 children aged between 4 and 19 years.
 Study investigators evaluated the four top selling antipsychotic medications used in the treatment of schizophrenia and bipolar disorder, namely Zyprexa (Eli Lilly & Co); Abilify, (Bristol-Myers Squibb Co and Otsuka Pharmaceutical Co); Risperdal (Johnson & Johnson); and Seroquel ( AstraZeneca PLC).
Study investigators evaluated the four top selling antipsychotic medications used in the treatment of schizophrenia and bipolar disorder, namely Zyprexa (Eli Lilly & Co); Abilify, (Bristol-Myers Squibb Co and Otsuka Pharmaceutical Co); Risperdal (Johnson & Johnson); and Seroquel ( AstraZeneca PLC).
Of the four atypical antipsychotics, Zyprexa caused the most weight gain over the course of the study – at nearly 19 lbs, a 15 percent increase. Importantly, Zyprexa was also found to significantly raise cholesterol, insulin, triglycerides and blood sugar levels, all of which can lead to the development of diabetes and heart disease.
Children taking Abilify, Risperdal, and Seroquel gained an average of between 10 and 13 pounds over the 11 weeks, and the drugs' impact on metabolic disease indicators varied.
In an article published in the Wall Street Journal (wsj.com), Christoph Correll, the study's lead author and a psychiatrist and scientist at the Feinstein Institute for Medical Research in Manhasset, NY, said, "The weight gain is much larger than we thought. It's massive, and it's the medication that caused it."
 Study investigators evaluated the four top selling antipsychotic medications used in the treatment of schizophrenia and bipolar disorder, namely Zyprexa (Eli Lilly & Co); Abilify, (Bristol-Myers Squibb Co and Otsuka Pharmaceutical Co); Risperdal (Johnson & Johnson); and Seroquel ( AstraZeneca PLC).
Study investigators evaluated the four top selling antipsychotic medications used in the treatment of schizophrenia and bipolar disorder, namely Zyprexa (Eli Lilly & Co); Abilify, (Bristol-Myers Squibb Co and Otsuka Pharmaceutical Co); Risperdal (Johnson & Johnson); and Seroquel ( AstraZeneca PLC).Of the four atypical antipsychotics, Zyprexa caused the most weight gain over the course of the study – at nearly 19 lbs, a 15 percent increase. Importantly, Zyprexa was also found to significantly raise cholesterol, insulin, triglycerides and blood sugar levels, all of which can lead to the development of diabetes and heart disease.
Children taking Abilify, Risperdal, and Seroquel gained an average of between 10 and 13 pounds over the 11 weeks, and the drugs' impact on metabolic disease indicators varied.
In an article published in the Wall Street Journal (wsj.com), Christoph Correll, the study's lead author and a psychiatrist and scientist at the Feinstein Institute for Medical Research in Manhasset, NY, said, "The weight gain is much larger than we thought. It's massive, and it's the medication that caused it."
Legal Help Now - Aiuto Legale Adesso
#Zyprexa #Abilify #Risperdal #Seroquel #Obesità #Bambini
#Diabete #Ipetensione #JAMA #WSJ
